HEMOSTASIS COAGULATION ROUTINE REAGENTS INSTRUMENTS T-TAS®01 INSTRUMENT
T-TAS® 01
Microfluidic chip system (AR, PL and HD Chip) to quantify the thrombus formation process under total blood flow conditions.
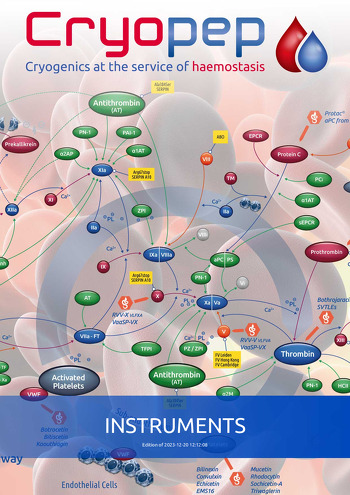
The T-TAS® 01 (Total Thrombus formation analysis system) provides a real-time, comprehensive, ex vivo assessment of blood haemostatic capacity.
It is composed of a portable instrument, a dedicated computer and a chip integrating chambers of flow covered with either collagen (PL Chip) to evaluate the primary hemostatic capacity (case of antiplatelet therapies or congenital platelet disorders),
a mixture of collagen and thromboplastin to assess primary and secondary hemostatic capacity (risk of bleeding) when platelet count is normal (AR Chip) or (HD Chip) if platelets are between 10,000 and 90,000/µL of blood to assess the risk of bleeding for thrombocytopenia and platelet transfusions.
Advantages
T-TAS® 01 is conformed with IVD CE marking.
Total thrombus formation analysis system for clinical use.
Single-use microchip produced by a precision injection molding technique requires only small-volume whole blood samples (approx. 320 μl).
Simple operation controlled by a dedicated computer system.
Informations
The formation of the platelet thrombus is a direct indicator of the primary hemostatic capacity of patients.
This test is performed under arterial flow conditions using whole blood samples anti-coagulated with benzylsulfonyl-D-ArgPro-4-amidinobenzylamide (BAPA).
BAPA is an anticoagulant that inhibits thrombin and Factor Xa, which blocks the coagulation cascade and allows the PL Chip test to specifically measure platelet thrombus formation (primary hemostasis).
Documentation
Download the product sheetPrice list, safety data sheets and notices are accessible to our registered customers.

Analyzers




References
| 25-18001 | Instrument | 1 |